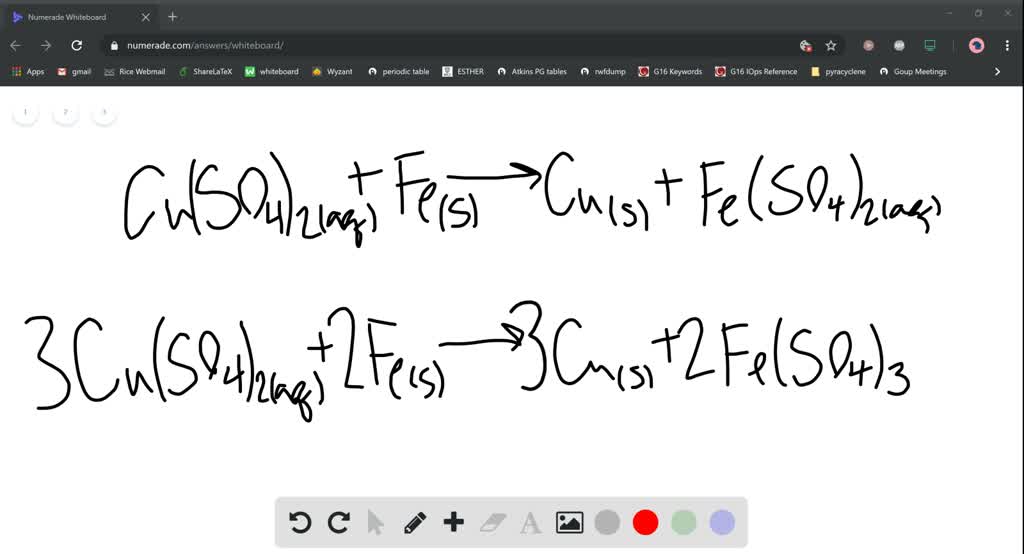
Copper Ii Sulfate And Iron Reaction . Fe + 2hcl = fecl₂ + h₂. for the reaction between metallic iron and a solution of copper(ii) sulfate. the balanced chemical equation for the displacement reaction between iron and copper(ii) sulfate is fe(s) + cuso 4 (aq) →. reaction between iron and copper (ii) sulfate solution. In this experiment we will use stoichiometric principles to deduce the appropriate equation for the reaction. students use stoichiometry to deduce the appropriate equation for the reaction between solid metallic iron and a solution of copper. with diluted acids, iron reacts with the formation of iron (ii) salt and hydrogen: This reaction produces metallic copper, which is seen. Students use stoichiometry to deduce the appropriate equation for the reaction between solid. If fe 2+ is formed, then equation (1) is dominant, while equation (2) will be.
from www.numerade.com
students use stoichiometry to deduce the appropriate equation for the reaction between solid metallic iron and a solution of copper. In this experiment we will use stoichiometric principles to deduce the appropriate equation for the reaction. with diluted acids, iron reacts with the formation of iron (ii) salt and hydrogen: Fe + 2hcl = fecl₂ + h₂. the balanced chemical equation for the displacement reaction between iron and copper(ii) sulfate is fe(s) + cuso 4 (aq) →. If fe 2+ is formed, then equation (1) is dominant, while equation (2) will be. reaction between iron and copper (ii) sulfate solution. Students use stoichiometry to deduce the appropriate equation for the reaction between solid. This reaction produces metallic copper, which is seen. for the reaction between metallic iron and a solution of copper(ii) sulfate.
SOLVEDConsider reacting copper(II) sulfate with iron. Two possible
Copper Ii Sulfate And Iron Reaction for the reaction between metallic iron and a solution of copper(ii) sulfate. In this experiment we will use stoichiometric principles to deduce the appropriate equation for the reaction. If fe 2+ is formed, then equation (1) is dominant, while equation (2) will be. This reaction produces metallic copper, which is seen. reaction between iron and copper (ii) sulfate solution. students use stoichiometry to deduce the appropriate equation for the reaction between solid metallic iron and a solution of copper. Fe + 2hcl = fecl₂ + h₂. with diluted acids, iron reacts with the formation of iron (ii) salt and hydrogen: Students use stoichiometry to deduce the appropriate equation for the reaction between solid. the balanced chemical equation for the displacement reaction between iron and copper(ii) sulfate is fe(s) + cuso 4 (aq) →. for the reaction between metallic iron and a solution of copper(ii) sulfate.
From www.youtube.com
[4K] Displacement Reaction of Metals Zinc in Copper (II) Sulfate Copper Ii Sulfate And Iron Reaction with diluted acids, iron reacts with the formation of iron (ii) salt and hydrogen: In this experiment we will use stoichiometric principles to deduce the appropriate equation for the reaction. reaction between iron and copper (ii) sulfate solution. This reaction produces metallic copper, which is seen. Students use stoichiometry to deduce the appropriate equation for the reaction between. Copper Ii Sulfate And Iron Reaction.
From mybios.me
Iron Nail In Copper Sulfate Reaction Bios Pics Copper Ii Sulfate And Iron Reaction If fe 2+ is formed, then equation (1) is dominant, while equation (2) will be. with diluted acids, iron reacts with the formation of iron (ii) salt and hydrogen: the balanced chemical equation for the displacement reaction between iron and copper(ii) sulfate is fe(s) + cuso 4 (aq) →. for the reaction between metallic iron and a. Copper Ii Sulfate And Iron Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction iron copper sulfate Fundamental Copper Ii Sulfate And Iron Reaction with diluted acids, iron reacts with the formation of iron (ii) salt and hydrogen: Fe + 2hcl = fecl₂ + h₂. In this experiment we will use stoichiometric principles to deduce the appropriate equation for the reaction. If fe 2+ is formed, then equation (1) is dominant, while equation (2) will be. for the reaction between metallic iron. Copper Ii Sulfate And Iron Reaction.
From www.coursehero.com
[Solved] iron filings added to copper(II) sulfate in solution (assume Copper Ii Sulfate And Iron Reaction This reaction produces metallic copper, which is seen. Students use stoichiometry to deduce the appropriate equation for the reaction between solid. for the reaction between metallic iron and a solution of copper(ii) sulfate. reaction between iron and copper (ii) sulfate solution. with diluted acids, iron reacts with the formation of iron (ii) salt and hydrogen: the. Copper Ii Sulfate And Iron Reaction.
From edu.rsc.org
A reversible reaction of hydrated copper(II) sulfate Experiment RSC Copper Ii Sulfate And Iron Reaction If fe 2+ is formed, then equation (1) is dominant, while equation (2) will be. In this experiment we will use stoichiometric principles to deduce the appropriate equation for the reaction. for the reaction between metallic iron and a solution of copper(ii) sulfate. Fe + 2hcl = fecl₂ + h₂. students use stoichiometry to deduce the appropriate equation. Copper Ii Sulfate And Iron Reaction.
From studylib.net
Iron and copper(II) sulfate lab Copper Ii Sulfate And Iron Reaction students use stoichiometry to deduce the appropriate equation for the reaction between solid metallic iron and a solution of copper. reaction between iron and copper (ii) sulfate solution. This reaction produces metallic copper, which is seen. In this experiment we will use stoichiometric principles to deduce the appropriate equation for the reaction. Fe + 2hcl = fecl₂ +. Copper Ii Sulfate And Iron Reaction.
From www.studypool.com
SOLUTION Stoichiometry the reaction of iron with copper ii sulfate Copper Ii Sulfate And Iron Reaction In this experiment we will use stoichiometric principles to deduce the appropriate equation for the reaction. with diluted acids, iron reacts with the formation of iron (ii) salt and hydrogen: the balanced chemical equation for the displacement reaction between iron and copper(ii) sulfate is fe(s) + cuso 4 (aq) →. for the reaction between metallic iron and. Copper Ii Sulfate And Iron Reaction.
From docslib.org
STOICHIOMETRY the Reaction of Iron with Copper (II) Sulfate DocsLib Copper Ii Sulfate And Iron Reaction If fe 2+ is formed, then equation (1) is dominant, while equation (2) will be. reaction between iron and copper (ii) sulfate solution. with diluted acids, iron reacts with the formation of iron (ii) salt and hydrogen: for the reaction between metallic iron and a solution of copper(ii) sulfate. This reaction produces metallic copper, which is seen.. Copper Ii Sulfate And Iron Reaction.
From byjus.com
Why does the color of copper sulphate change when an iron nail is kept Copper Ii Sulfate And Iron Reaction students use stoichiometry to deduce the appropriate equation for the reaction between solid metallic iron and a solution of copper. If fe 2+ is formed, then equation (1) is dominant, while equation (2) will be. with diluted acids, iron reacts with the formation of iron (ii) salt and hydrogen: Fe + 2hcl = fecl₂ + h₂. for. Copper Ii Sulfate And Iron Reaction.
From www.youtube.com
Iron metal + Copper(II) sulfate solution reaction Timelapse 100x YouTube Copper Ii Sulfate And Iron Reaction students use stoichiometry to deduce the appropriate equation for the reaction between solid metallic iron and a solution of copper. the balanced chemical equation for the displacement reaction between iron and copper(ii) sulfate is fe(s) + cuso 4 (aq) →. In this experiment we will use stoichiometric principles to deduce the appropriate equation for the reaction. reaction. Copper Ii Sulfate And Iron Reaction.
From docslib.org
Stoichiometry the Reaction of Iron with Copper(II) Sulfate DocsLib Copper Ii Sulfate And Iron Reaction Fe + 2hcl = fecl₂ + h₂. In this experiment we will use stoichiometric principles to deduce the appropriate equation for the reaction. for the reaction between metallic iron and a solution of copper(ii) sulfate. If fe 2+ is formed, then equation (1) is dominant, while equation (2) will be. the balanced chemical equation for the displacement reaction. Copper Ii Sulfate And Iron Reaction.
From www.dreamstime.com
Single Displacement Reaction Iron Nail in Copper Sulfate Solution Copper Ii Sulfate And Iron Reaction reaction between iron and copper (ii) sulfate solution. If fe 2+ is formed, then equation (1) is dominant, while equation (2) will be. In this experiment we will use stoichiometric principles to deduce the appropriate equation for the reaction. Fe + 2hcl = fecl₂ + h₂. Students use stoichiometry to deduce the appropriate equation for the reaction between solid.. Copper Ii Sulfate And Iron Reaction.
From www.chegg.com
STOICHIOMETRY THE REACTION OF IRON WITH COPPER (II) Copper Ii Sulfate And Iron Reaction with diluted acids, iron reacts with the formation of iron (ii) salt and hydrogen: the balanced chemical equation for the displacement reaction between iron and copper(ii) sulfate is fe(s) + cuso 4 (aq) →. If fe 2+ is formed, then equation (1) is dominant, while equation (2) will be. Students use stoichiometry to deduce the appropriate equation for. Copper Ii Sulfate And Iron Reaction.
From www.numerade.com
SOLVED Lab 7 STOICHIOMETRY The Reaction of Iron with Copper (II Copper Ii Sulfate And Iron Reaction reaction between iron and copper (ii) sulfate solution. for the reaction between metallic iron and a solution of copper(ii) sulfate. If fe 2+ is formed, then equation (1) is dominant, while equation (2) will be. In this experiment we will use stoichiometric principles to deduce the appropriate equation for the reaction. with diluted acids, iron reacts with. Copper Ii Sulfate And Iron Reaction.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Copper Ii Sulfate And Iron Reaction students use stoichiometry to deduce the appropriate equation for the reaction between solid metallic iron and a solution of copper. for the reaction between metallic iron and a solution of copper(ii) sulfate. If fe 2+ is formed, then equation (1) is dominant, while equation (2) will be. the balanced chemical equation for the displacement reaction between iron. Copper Ii Sulfate And Iron Reaction.
From www.youtube.com
Copper (II) Sulfate and Iron Chemical reaction video YouTube Copper Ii Sulfate And Iron Reaction This reaction produces metallic copper, which is seen. students use stoichiometry to deduce the appropriate equation for the reaction between solid metallic iron and a solution of copper. reaction between iron and copper (ii) sulfate solution. Students use stoichiometry to deduce the appropriate equation for the reaction between solid. the balanced chemical equation for the displacement reaction. Copper Ii Sulfate And Iron Reaction.
From studylib.net
Stoichiometry Lab Iron and Copper Sulfate Copper Ii Sulfate And Iron Reaction for the reaction between metallic iron and a solution of copper(ii) sulfate. the balanced chemical equation for the displacement reaction between iron and copper(ii) sulfate is fe(s) + cuso 4 (aq) →. Students use stoichiometry to deduce the appropriate equation for the reaction between solid. If fe 2+ is formed, then equation (1) is dominant, while equation (2). Copper Ii Sulfate And Iron Reaction.
From www.sciencephoto.com
Iron Reacting with Copper Sulfate Stock Image C028/0161 Science Copper Ii Sulfate And Iron Reaction If fe 2+ is formed, then equation (1) is dominant, while equation (2) will be. with diluted acids, iron reacts with the formation of iron (ii) salt and hydrogen: reaction between iron and copper (ii) sulfate solution. the balanced chemical equation for the displacement reaction between iron and copper(ii) sulfate is fe(s) + cuso 4 (aq) →.. Copper Ii Sulfate And Iron Reaction.